Definitions for the complementary processes of this reaction class are correspondingly revised as shown here: oxidation reduction = increase in oxidation number = decrease in oxidation number (6.5.9) (6.5.10) Returning to the reactions used to introduce this topic, they may now both be identified as redox processes.
Scale-up of bi-phasic fast competitive oxidation reaction – Mixing Solution
Preview Balancing Oxidation-Reduction Equations Assignment Teacher 8 terms cjewels34 Preview Redox Reactions Unit Test Review and Test 100% 40 terms katherinenguyenn Preview Chemical Kinetics 13 terms AlOK616 Preview
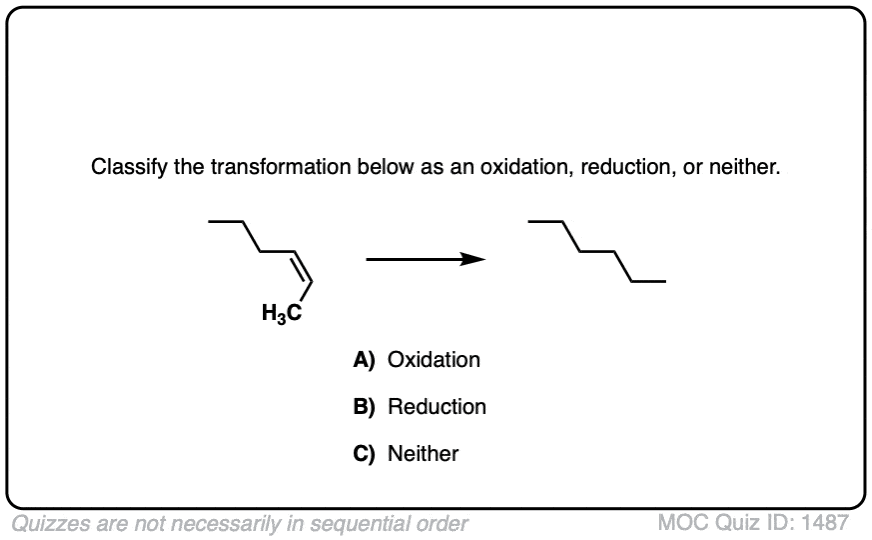
Source Image: masterorganicchemistry.com
Download Image
The corrosion process involves an oxidation-reduction reaction in which metallic iron is converted to Fe (OH) 3, a reddish-brown solid. Many metals dissolve through reactions of this type, which have the general form. metal + acid → salt + hydrogen (4.9.8) (4.9.8) metal + acid → salt + hydrogen.

Source Image: studocu.com
Download Image
Oxidation & Reduction IB Topics 9 & 19 AP Chapters ; ppt download The term oxidation was originally used to describe chemical reactions involving O 2, but its meaning has evolved to refer to a broad and important reaction class known as oxidation-reduction (redox) reactions. The most easily identified redox reactions involve the exchange of electrons between reactants.
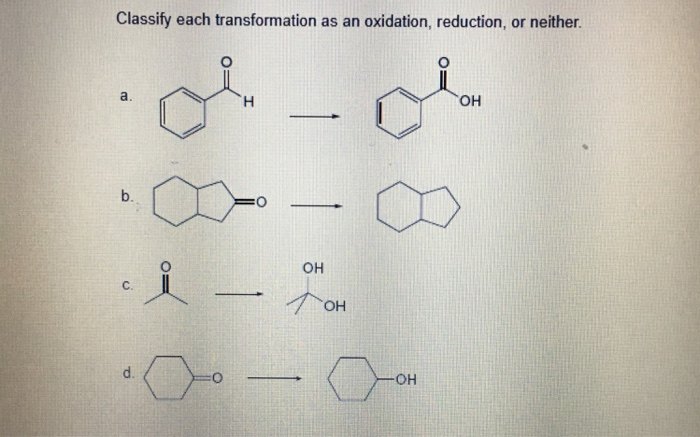
Source Image: chegg.com
Download Image
Which Of The Following Reactions Would Be Classified As Oxidation-Reduction
The term oxidation was originally used to describe chemical reactions involving O 2, but its meaning has evolved to refer to a broad and important reaction class known as oxidation-reduction (redox) reactions. The most easily identified redox reactions involve the exchange of electrons between reactants. Science Chemistry Chemistry questions and answers Which of the following reactions would be classified as oxidation-reduction? Check all that apply. View Available Hint (s) O2Na (s) + Cl₂ (9) 2NaCl (s) Na (s) + CuCl (aq)→NaCl (aq) + Cu (s) _ NaCN (aq)+CuCl (aq)—NaCl (aq)+CuCN (s) Submit Request Answer 2012 PERPU S This problem has been solved!
Solved Classify each transformation as an oxidation, | Chegg.com
An oxidation-reduction or redox reaction is a reaction that involves the transfer of electrons between chemical species (the atoms, ions, or molecules involved in the reaction). The duality of moonlighting GAPDH function | Download Scientific Diagram

Source Image: researchgate.net
Download Image
Chapter 6 Oxidation-Reduction Reactions An oxidation-reduction or redox reaction is a reaction that involves the transfer of electrons between chemical species (the atoms, ions, or molecules involved in the reaction).
Source Image: preparatorychemistry.com
Download Image
Scale-up of bi-phasic fast competitive oxidation reaction – Mixing Solution Definitions for the complementary processes of this reaction class are correspondingly revised as shown here: oxidation reduction = increase in oxidation number = decrease in oxidation number (6.5.9) (6.5.10) Returning to the reactions used to introduce this topic, they may now both be identified as redox processes.

Source Image: mixing-solution.com
Download Image
Oxidation & Reduction IB Topics 9 & 19 AP Chapters ; ppt download The corrosion process involves an oxidation-reduction reaction in which metallic iron is converted to Fe (OH) 3, a reddish-brown solid. Many metals dissolve through reactions of this type, which have the general form. metal + acid → salt + hydrogen (4.9.8) (4.9.8) metal + acid → salt + hydrogen.

Source Image: slideplayer.com
Download Image
Solved QUESTION 1 Is the following reaction classified as | Chegg.com Also called: oxidation-reduction reaction, any chemical reaction in which the oxidation number of a participating chemical species changes. The term covers a large and diverse body of processes. Many oxidation– reduction reactions are as common and familiar as fire, the rusting and dissolution of metals, the browning of fruit, and respiration
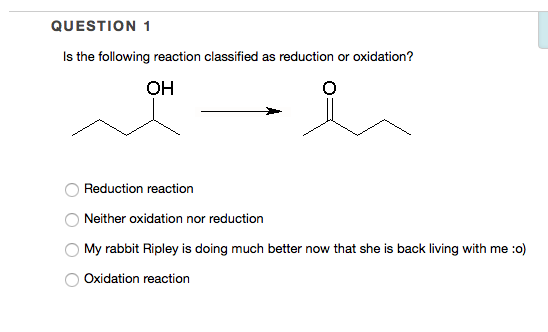
Source Image: chegg.com
Download Image
CH103 – Chapter 7: Chemical Reactions in Biological Systems – Chemistry The term oxidation was originally used to describe chemical reactions involving O 2, but its meaning has evolved to refer to a broad and important reaction class known as oxidation-reduction (redox) reactions. The most easily identified redox reactions involve the exchange of electrons between reactants.
Source Image: wou.edu
Download Image
Oxidation and Reduction reactions- Definition, Reactions, Examples, Balancing the oxidation and reduction reactions, Videos and FAQs Science Chemistry Chemistry questions and answers Which of the following reactions would be classified as oxidation-reduction? Check all that apply. View Available Hint (s) O2Na (s) + Cl₂ (9) 2NaCl (s) Na (s) + CuCl (aq)→NaCl (aq) + Cu (s) _ NaCN (aq)+CuCl (aq)—NaCl (aq)+CuCN (s) Submit Request Answer 2012 PERPU S This problem has been solved!

Source Image: byjus.com
Download Image
Chapter 6 Oxidation-Reduction Reactions
Oxidation and Reduction reactions- Definition, Reactions, Examples, Balancing the oxidation and reduction reactions, Videos and FAQs Preview Balancing Oxidation-Reduction Equations Assignment Teacher 8 terms cjewels34 Preview Redox Reactions Unit Test Review and Test 100% 40 terms katherinenguyenn Preview Chemical Kinetics 13 terms AlOK616 Preview
Oxidation & Reduction IB Topics 9 & 19 AP Chapters ; ppt download CH103 – Chapter 7: Chemical Reactions in Biological Systems – Chemistry Also called: oxidation-reduction reaction, any chemical reaction in which the oxidation number of a participating chemical species changes. The term covers a large and diverse body of processes. Many oxidation– reduction reactions are as common and familiar as fire, the rusting and dissolution of metals, the browning of fruit, and respiration