Step by step Solved in 3 steps SEE SOLUTION Check out a sample Q&A here Similar questions What mass of a 4.00% NaOH solution by mass contains 15.0 g of NaOH? A cough syrup contains 5.0% ethyl alcohol, C2H5OH, by mass. If the density of the solution is 0.9928 g/mL, determine the molarity of the alcohol in the cough syrup.
Prof. Anand Dhutraj – N. L. Dalmia Institute of Management Studies and Research
What volume in L of a 0.724 M Nal solution contains 0.405 mol of Nal ? х х STARTING AMOUNT ADD FACTOR ANSWER RESET * ( ) 1 559 M Nal 0.405 0.559 L 1.79 22.99 g Nal/mol 0.724 148.89 ml 126.90 mol Nal 6.022 x 1023 g Nal 0.293 This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts.

Source Image: pinterest.com
Download Image
What volume in L of a 0.724 M Nal solution contains 0.405 mol of Nal ? BUY Introductory Chemistry: A Foundation 9th Edition ISBN: 9781337399425 Author: Steven S. Zumdahl, Donald J. DeCoste Publisher: Cengage Learning expand_more expand_more format_list_bulleted Concept explainers Question
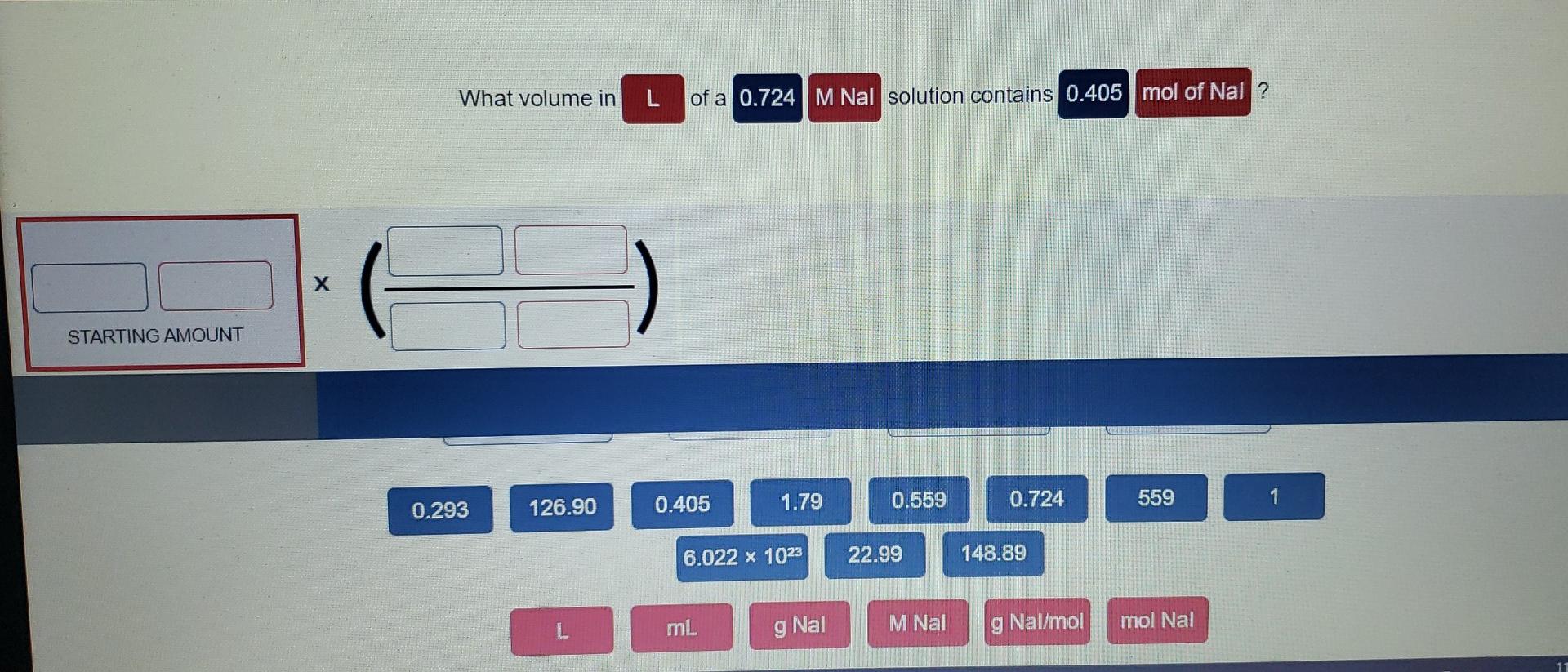
Source Image: chegg.com
Download Image
Solved What quantity of moles of glucose (C6H12O6) in 0.500 | Chegg.com Knowledge Booster Learn more about Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below. Similar questions arrow_back_ios arrow_forward_ios

Source Image: numerade.com
Download Image
What Volume In L Of A 0.724 M Nal
Knowledge Booster Learn more about Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below. Similar questions arrow_back_ios arrow_forward_ios May 31, 2022ADD FACTOR ANSWER RESET 0.405 mol 1.79 g/mol 0.724 M 6.022 x 10^23 molecules/mol 559 mL 22.99 g/mol 148.89 g 0.559 L 126.90 g/mol 0.293 g NaCl mL
SOLVED: What volume in L of a 0.724 M Nal solution contains 0.405 mol of Nal ? 4 0 of 1 point earned -0.2 points (incorrect attempts) attempt remaining
verified answered • expert verified What volume in L of a 0.724 M Nal solution contains 0.405 mol of Nal ? Expert-Verified Answer %counter% person found it helpful boffeemadrid 1 Answer: Explanation: Mol of NaI = 0.405 mol Molarity of solution = 0.724 M Molarity is given by The required volume is . .559 is the answer report flag outlined Solved 2. Calculate the volume of a 0.850 M KOH solution | Chegg.com
Source Image: chegg.com
Download Image
What volume in L of a 0.724 M Nal solution contains 0.405 mol of Nal? – brainly.com verified answered • expert verified What volume in L of a 0.724 M Nal solution contains 0.405 mol of Nal ? Expert-Verified Answer %counter% person found it helpful boffeemadrid 1 Answer: Explanation: Mol of NaI = 0.405 mol Molarity of solution = 0.724 M Molarity is given by The required volume is . .559 is the answer report flag outlined

Source Image: brainly.com
Download Image
Prof. Anand Dhutraj – N. L. Dalmia Institute of Management Studies and Research Step by step Solved in 3 steps SEE SOLUTION Check out a sample Q&A here Similar questions What mass of a 4.00% NaOH solution by mass contains 15.0 g of NaOH? A cough syrup contains 5.0% ethyl alcohol, C2H5OH, by mass. If the density of the solution is 0.9928 g/mL, determine the molarity of the alcohol in the cough syrup.

Source Image: nldalmia.in
Download Image
Solved What quantity of moles of glucose (C6H12O6) in 0.500 | Chegg.com What volume in L of a 0.724 M Nal solution contains 0.405 mol of Nal ? BUY Introductory Chemistry: A Foundation 9th Edition ISBN: 9781337399425 Author: Steven S. Zumdahl, Donald J. DeCoste Publisher: Cengage Learning expand_more expand_more format_list_bulleted Concept explainers Question
Source Image: chegg.com
Download Image
The Plaid Horse- January 2023 by The Plaid Horse – Issuu Mar 21, 2023Expert-Verified Answer question No one rated this answer yet — why not be the first? 😎 saishreechandan907 A 0.724 M Nal solution with 0.405 mol of Nal has a volume of 0.559 L. Reason: The following equation can be used to determine the volume of a solution: Volume is calculated as moles of solute divided by the molarity of the solution.

Source Image: issuu.com
Download Image
measurement visual meters liters grams | Volume worksheets, Capacity worksheets, Math measurement Knowledge Booster Learn more about Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below. Similar questions arrow_back_ios arrow_forward_ios

Source Image: ru.pinterest.com
Download Image
Answered: What volume in L of a 0.724|M Nal… | bartleby May 31, 2022ADD FACTOR ANSWER RESET 0.405 mol 1.79 g/mol 0.724 M 6.022 x 10^23 molecules/mol 559 mL 22.99 g/mol 148.89 g 0.559 L 126.90 g/mol 0.293 g NaCl mL
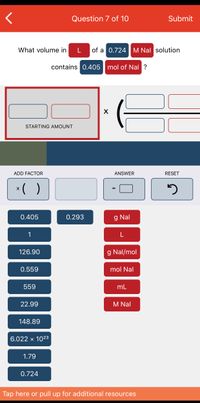
Source Image: bartleby.com
Download Image
What volume in L of a 0.724 M Nal solution contains 0.405 mol of Nal? – brainly.com
Answered: What volume in L of a 0.724|M Nal… | bartleby What volume in L of a 0.724 M Nal solution contains 0.405 mol of Nal ? х х STARTING AMOUNT ADD FACTOR ANSWER RESET * ( ) 1 559 M Nal 0.405 0.559 L 1.79 22.99 g Nal/mol 0.724 148.89 ml 126.90 mol Nal 6.022 x 1023 g Nal 0.293 This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts.
Solved What quantity of moles of glucose (C6H12O6) in 0.500 | Chegg.com measurement visual meters liters grams | Volume worksheets, Capacity worksheets, Math measurement Mar 21, 2023Expert-Verified Answer question No one rated this answer yet — why not be the first? 😎 saishreechandan907 A 0.724 M Nal solution with 0.405 mol of Nal has a volume of 0.559 L. Reason: The following equation can be used to determine the volume of a solution: Volume is calculated as moles of solute divided by the molarity of the solution.